Microalgae are relatively simple photosynthetic organisms that can be used to elucidate the fundamental and evolutionarily conserved mechanisms by which photosynthesis is regulated. Similar to plants, the unicellular green alga Chromochloris zofngiensis reversibly shuts off photosynthesis in the presence of the sugar glucose. In C. zofngiensis glucose also triggers rapid accumulation of the biofuel precursor TAG and the high-value antioxidant astaxanthin, making it a promising candidate organism for bioproduction. Insight into how photosynthesis and metabolism are regulated will enable rerouting and engineering of metabolism to improve commercial prospects of plant and algal bioproducts.
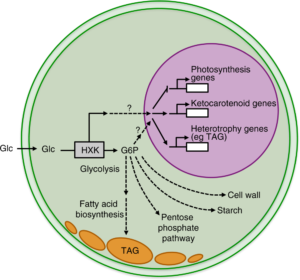
Model of hexokinase-dependent glucose pathways in C. zofingiensis.
Using a forward genetics approach, a team of Biosciences researchers revealed that the enzyme hexokinase (HXK1), which is involved in sugar metabolism in organisms ranging from bacteria to plants to humans, is necessary for the photosynthetic and metabolic switch in C. zofngiensis. Nutrients such as glucose play essential regulatory roles in gene expression, metabolism, growth and aging in plants, animals, yeast and bacteria. By elucidating HXK signaling in C. zofingiensis, a simpler system at the base of the green lineage, this study provides insights into fundamental and evolutionarily conserved mechanisms of glucose signaling and regulation of photosynthesis.
Kris Niyogi, a faculty scientist in Molecular Biophysics and Integrated Bioimaging (MBIB), was senior author on the paper, published in Nature Communications Biology. Melissa Roth, a former postdoctoral fellow in Niyogi’s lab who is now an assistant researcher at UC Berkeley (UCB), and Daniel Westcott, graduate student in the Niyogi lab, were co-first authors. Masakazu Iwai, a research scientist in MBIB, also contributed to the study.




