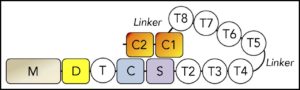
Human ADAMTS13 is a multidomain protein with metalloprotease (M), disintegrin-like (D), thrombospondin-1 (T), Cys-rich (C), and spacer (S) domains, followed by 7 additional T domains and 2 CUB domains (a structural motif of ~110 amino acids).
A group of researchers at Washington University School of Medicine have used the capabilities available at the Advanced Light Source’s SIBYLS beamline to gain insight into an enzyme that functions in blood clotting. ADAMTS13 (which stands for a disintegrin and metalloproteinase with thrombospondin-1 repeats, member 13) is a multi-domain protease enzyme whose catalytic mechanism involves a metal. It is the only known protein to regulate the adhesive function of von Willebrand factor (VWF), a blood clotting protein involved in hemostasis. When VWF is deficient or abnormal, it causes a common inherited bleeding disorder, von Willebrand disease. There is increasing evidence that VWF is implicated in several other disorders as well, including arterial and deep vein thrombosis, stroke, atherosclerosis, sickle cell crisis, and sepsis.
The inhibition of VWF-platelet aggregate growth by ADAMTS13 is regulated by substrate-induced allosteric activation, i.e., a conformational change upon the binding of a molecule. To gain insight into the molecular mechanism of this allosteric regulation, researchers used small angle X-ray scattering (SAXS) on SIBYLS beamline 12.3.1 at the Advanced Light Source (ALS). SAXS is a useful approach to obtain low-resolution (10-50 Å) structures under physiological conditions of pH and ionic strength for a flexible protein such as ADAMTS13 that is not amenable to crystallization or electron microscopy.
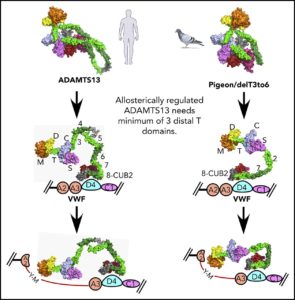 SIBYLS beamline scientist Greg Hura, research assistant Kathryn Burnett, and research technician Kevin Dyer collected SAXS data that was used to characterize the molecular envelopes of native ADAMTS13, as well as truncated constructs with different configurations of its distal thrombospondin-1 (T) domains. Previous findings by the study’s first author, Jian Zhu, suggested that the distal T domains partially interfere with the proximal domains to maintain the ADAMTS13 molecule in an auto-inhibited state.
SIBYLS beamline scientist Greg Hura, research assistant Kathryn Burnett, and research technician Kevin Dyer collected SAXS data that was used to characterize the molecular envelopes of native ADAMTS13, as well as truncated constructs with different configurations of its distal thrombospondin-1 (T) domains. Previous findings by the study’s first author, Jian Zhu, suggested that the distal T domains partially interfere with the proximal domains to maintain the ADAMTS13 molecule in an auto-inhibited state.
The results of the current study, published in the journal Blood and featured on the cover of the April 25, 2019 issue, provided evidence that ADAMTS13 folds roughly in half to allow formation of an allosterically regulated complex of MDTCS domains, T7, T8, and CUB1. The researchers were also able to determine the minimal structure of ADAMTS13 that preserves its proteolytic activity on VWF. This improved understanding is crucial for the possible development of ADAMTS13 as a therapeutic target to treat VWF-related thrombotic disorders.
The team of researchers was led by Evan Sadler, director of the Division of Hematology at Washington University School of Medicine, who passed away following a brief illness prior to this study’s publication.




