Photosynthesis, the process by which green plants (as well as some bacteria and other organisms) harness energy from the sun to transform water and carbon dioxide into oxygen and energy-rich organic compounds, is responsible for producing all of the air that we breathe and most of the food that we eat. It’s a pretty efficient system, but even so, plants can at best utilize only about a third of the sunlight that reaches them under optimal conditions.
Photosynthesis, the process by which green plants (as well as some bacteria and other organisms) harness energy from the sun to transform water and carbon dioxide into oxygen and energy-rich organic compounds, is responsible for producing all of the air that we breathe and most of the food that we eat. It’s a pretty efficient system, but even so, plants can at best utilize only about a third of the sunlight that reaches them under optimal conditions.
Now, work by Berkeley Lab scientists reported in pair of recent papers provides several key insights into the mechanisms underlying one type of photoprotection. When plants absorb more light energy than they can use—such as during a brief period of intense illumination—they have mechanisms to dissipate the excess energy as heat, thereby avoiding damage to their light-harvesting pigment complexes. But this essential protective response, which is known as non-photochemical quenching, comes with an opportunity cost, explained Graham Fleming, a senior faculty scientist in Molecular Biophysics and Integrated Bioimaging.
Research has suggested that engineering plants’ non-photochemical quenching capabilities to minimize productivity loss could increase crop yields by up to 30 percent. And that would go a long way toward meeting future global food demand. However, significant gaps remain in scientists’ understanding of the molecular details underlying these mechanisms, including how they are triggered and their activation dynamics.
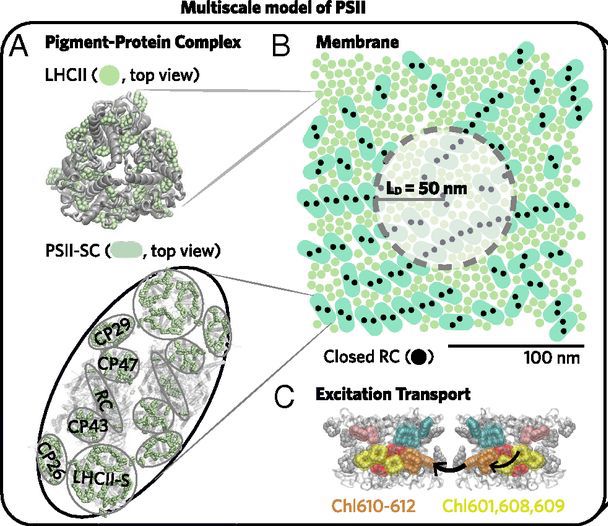
In order to fill in these gaps, the scientists focused on photosystem II, a protein complex located in the thylakoid membrane of plants, algae, and cyanobacteria, which kicks off the light-dependent reactions of photosynthesis. Doran Bennett and Kapil Amarnath, both former students in Fleming’s lab in the UC Berkeley department of chemistry, were co-authors on one paper published in the Proceedings of the National Academy of Sciences (PNAS), which used theoretical methods to study the how photosystem II responds to too much light.
During exposure to excess sunlight, a plant’s capacity to fix carbon dioxide is maxed out and photosynthetic electron transport causes hydrogen ions to build up within thylakoid lumen, the compartment enclosed by the thylakoid membrane. This creates a pH gradient across the membrane that triggers a mechanism to temporarily quell over-excitation.
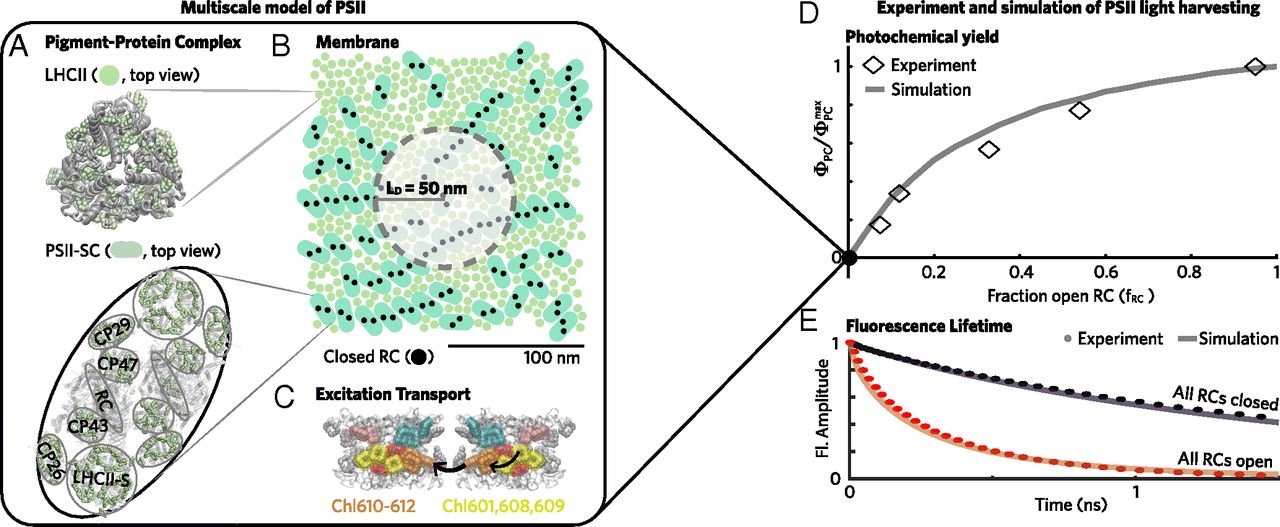
Energy-dependent quenching of “excitations” (that is, the state of an antenna protein such as the green pigment chlorophyll that has absorbed light energy) comprises the largest portion of non-photochemical quenching; it occurs on the seconds to minutes time scale and is rapidly reversible. Using a multi-scale model of photosystem II, Fleming and colleagues demonstrated that just one parameter—the excitation diffusion distance—accounts for the down regulation in light harvesting due to energy-dependent quenching.
“Elucidating the mechanisms that connect excitation transport and quenching in proteins to chlorophyll fluorescence and photosystem II productivity provides an avenue to quantitatively explore how specific mutations, for example, influence photosynthetic yield,” said Fleming. “It’s remarkable that such a complex problem can be described by a single quantity.”
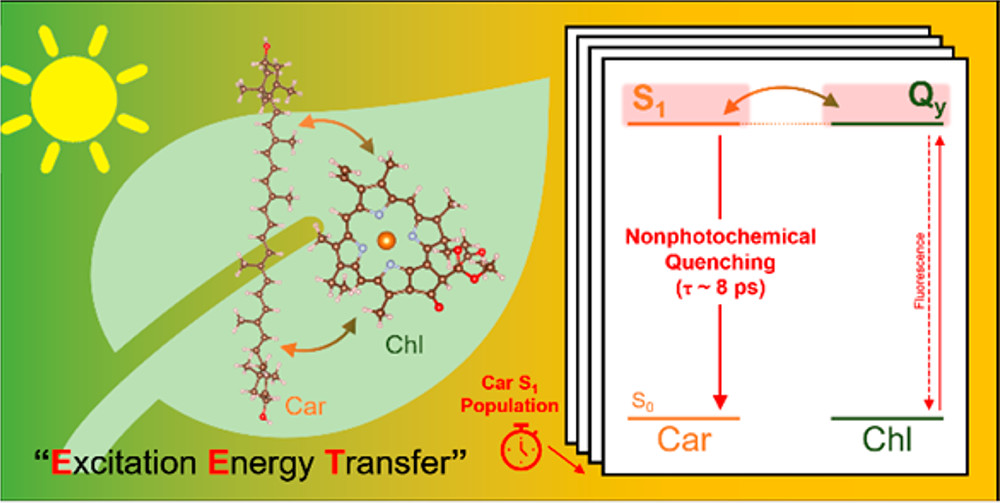
In the second paper, published in the Journal of the American Chemical Society (JACS), co-first authors Soomin Park and Alexandra Fischer—a postdoctoral researcher and a graduate student, respectively, in Fleming’s lab—used a new technique called snapshot transient absorption spectroscopy to monitor the interaction of chlorophyll and zeaxanthin (another antenna pigment) in intact thylakoid membranes from spinach. This novel method enabled them to gain insight into the molecular actors and mechanisms responsible for dissipation of the excess energy. The two key mechanisms are excitation energy transfer and charge transfer, both involving the zeaxanthin molecule which plays a central role in energy-dependent quenching in vivo.
“One metaphor I really like to explain energy-dependent quenching is the fire extinguisher and the fire engine. When the fire breaks out, the fire extinguisher can be easily accessible so it can be a fast response until the fire engine arrives,” said Park. “When plants are suddenly exposed to high light intensity they need to relieve their oxidative stress, so by taking advantage of these two mechanisms energy-dependent quenching can serve as a fire extinguisher until other, longer time scale mechanisms can be activated.”
Fleming led the team that studied energy-dependent quenching using snapshot transient absorption spectroscopy. Additional MBIB collaborators were: faculty scientist Krishna Niyogi, research scientist Masakazu Iwai, and graduate students Collin Steen and Jonathan Morris. This research was supported by the DOE Office of Science.