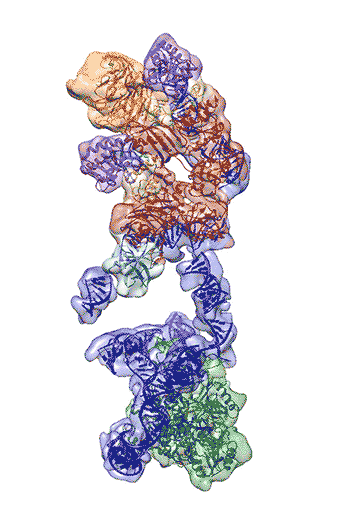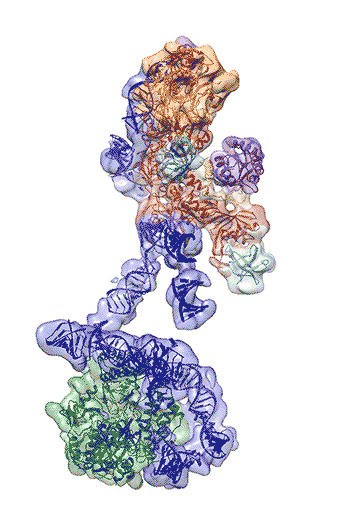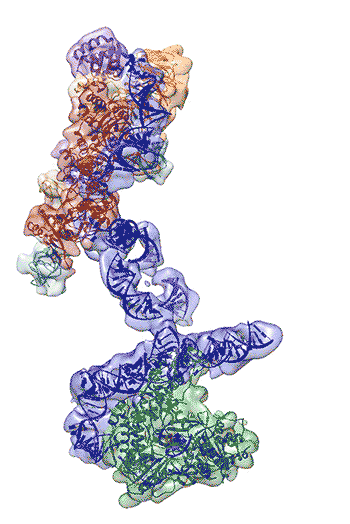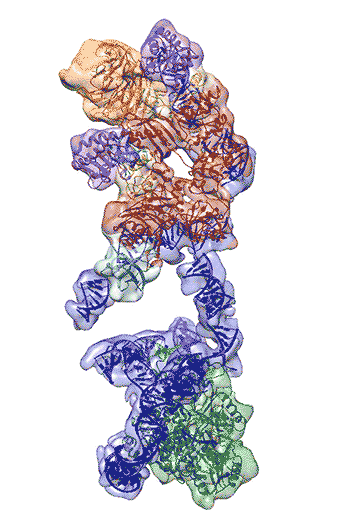
Regulation of the enzyme telomerase has been implicated in cancer, other human diseases, and aging, but progress towards therapeutic manipulation of telomerase has been hampered by the lack of high-resolution structural data. Now, researchers in senior faculty scientist Eva Nogales’ lab in Molecular Biophysics and Integrated Bioimaging (MBIB), in collaboration with UC Berkeley professor of Biochemistry, Biophysics and Structural Biology Kathleen Collins, have published a paper in Nature describing the 3-D molecular structure of the human telomerase enzyme. The paper was featured on the NIH Director’s blog, and Collins and Nogales were quoted in Newsweek, among other media coverage.
Telomeres, the protective structures at the ends of each arm of a chromosome, have been likened to the plastic tips at the ends of shoelaces: They prevent the DNA strands from “fraying” to the point that they become unable to perform their essential function. Every time a cell replicates, though, the telomeres get progressively shorter. And without their protection, the cell stops working properly, eventually becoming senescent or dying. In embryonic cells, telomerase counteracts this process, rebuilding the telomeres by adding repetitive nucleotide sequences to the chromosome ends, effectively extending the cell’s lifespan. However, in humans and other multicellular organisms telomerase is not expressed in most adult cells.
First author Kelly Nguyen, a postdoctoral fellow in Nogales’s group, was able to isolate the active enzyme and purify it far better than anyone previously had. The researchers then employed a new, state-of-the-art cryo-electron microscope to determine the structure of the active telomerase unambiguously. The newly revealed structure still lacks fine detail, but combined with knowledge of the gene sequence of human telomerase, it provides enough information to start thinking about potential targets for drugs, Nguyen said. She, Collins, and Nogales are actively working to improve the resolution to 3 or 4 Ångstroms ,which is sufficient for drug design.
Read more in the UC Berkeley News Center.