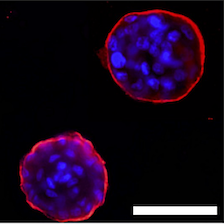
Findings shed new light on how the extracellular matrix communicates with breast cells to generate nitric oxide, forming a loop that influences the pathway a single cell takes to form breast tissue.
Once the egg and sperm unite in a fertilized egg, a new genetic code made of pieces of DNA inherited from the parents determine the sex and the unique DNA of the individual. Despite the fact that the same DNA will be duplicated in all the cells of the body as the embryo grows, each tissue and organ, e.g. liver and mammary gland, have an entirely different appearance and architecture, or what is referred to as ‘phenotype’. The phenotype arises because cells in our tissues exist in a microenvironment consisting of other cells and the extracellular matrix (ECM), which provides the context that helps determine their form as well as their function. A team of scientists led by Mina Bissell, distinguished scientist in the Biological Systems and Engineering (BSE) Division at Lawrence Berkeley National Laboratory (Berkeley Lab), has proven that the signaling between the ECM and the nucleus is pivotal, bidirectional, and intricate.

Mina Bissell
Berkeley Lab Distinguished Scientist
In a March 21 report published in eLife, Bissell and first author Saori Furuta, a former postdoctoral researcher in Bissell’s lab who is now an assistant professor at the University of Toledo, elucidate their search for the common regulators of these diverse signaling pathways. They began by growing cells that build breast tissue and glands in 3D gels containing an ECM protein called laminin111 (LN1). In the 3D ECM gels, the ‘normal’ cells form organized clusters (organoids) that resemble breast glands whereas breast cancer cells form structures that resemble tumors.
Building on four decades of research, Bissell and her team have demonstrated a dynamic reciprocity between the ECM and cell nucleus for tissue-specific gene expression. Using the 3D ECM gel to study signaling from outside the cell to the nucleus they have unraveled a dozen different pathways critical for the formation of phenotypically normal breast tissue. They showed also that when each of these pathways fire at high levels due to mutations or changes in the microenvironment, the balance that allows cells to form healthy structures is disturbed and the resulting structures resemble malignant tumors. Nevertheless, over the years, this team of scientists has demonstrated that, in spite of a multitude of genetic mutations in the DNA of cancer cells, reducing signaling of a number of these pathways individually to the level of normal cells could ‘revert’ the malignant cell phenotype so they would display ‘normal’ organoid characteristics in 3D gels when laminins (LNs) are present.

Saori Furuta
Assistant Professor, University of Toledo
“The fact that so many of the single pathways in these cancer cells could be reverted to form normal-like structures in 3D led us to postulate that there had to be common genes that regulate a number of the pathways,” Bissell explains. “Therefore, we set about identifying the genes that could be involved and found 60 that were being influenced by reverting agents.” Scientists found these genes by analyzing the pattern of expression in healthy, cancer, and phenotypically ‘reverted’ cancer cells, with those that were equally under-expressed in healthy and ‘reverted’ cells being the genes of interest.
In addition, they wanted to see if any small non-coding RNAs, called microRNAs, could be acting in this process. Typically 22 nucleotides in length, microRNAs (miRNAs) function in RNA silencing and post-transcriptional regulation of gene expression in plants, animals, and some viruses. They found ten miRNAs that potentially target these genes. A database search suggested that three of these miRNAs, which were downregulated or virtually absent in cancer cells, might be responsible for healthy breast cells to form organized structures.
While studying the signaling cascades involving these miRNAs, Bissell and her team identified a reciprocal regulatory network, comprising of LNs, nitric oxide (NO*), activated p53 tumor suppressor, proteolytic enzymes that cut the ECM, referred to as matrix metalloproteinases, and other transcription factors that form many connections between the ECM proteins, LNs, and the nuclear factors to execute breast tissue formation. “It is incredible,” says Bissell, “to find that NO* is essential for healthy structures since almost all studies with NO* were noted to make toxic reactive oxygen species that cause chromatin damage. But the amount of NO* required in breast tissue morphogenesis is drastically lower (> 500 fold lower) than used in the literature.” Another surprising discovery: tumor suppressor protein p53 needs to be present in normal tissues. Bissell says, “More than 90,000 papers have been written about p53, but very little work has been done to study the role of p53 in normal morphogenesis. We show here that not only it is present, but that it needs laminins to get activated.”
Delving into the process of cancer cell reversion to form ‘normal’ tissue architecture
The researchers had found previously that if the communication between healthy cells and the ECM is interrupted, the cells become disorganized, lose function, and start to form clumps that resemble and can behave like tumors. Bissell explains, “If the balance of signaling pathways between the microenvironment ECM and the cells is restored, the single cancer cells–despite all the mutations–can each be turned into healthy looking cells that form a normal-looking tissue in the presence of LNs, by a process called reversion.” Furuta adds, “Until now, it was not known which of the signaling molecules the laminins activate to help single ‘normal’ breast cells to form tissues, or how the cancer cells revert into a ‘normal’ phenotype.”
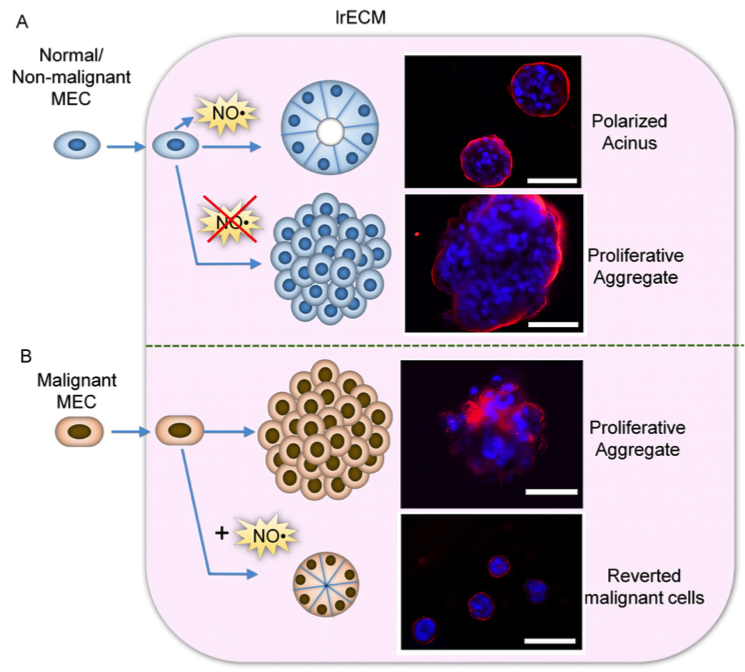
NO* production by mammary epithelial cells (MECs) in the presence of laminin-rich extracellular matric (lrECM) is critical for formation of polarized cell cluster, or acinar, structure. A. (Top) Non-malignant MECs produce NO* upon interaction with lrECM and forms acinar structures. (Bottom) Inhibition of NO* production by a specific inhibitor leads to formation of proliferative aggregates. B. (Top) Malignant MECs do not produce NO* in the presence of lrECM and forms proliferative aggregates. (Bottom) Addition of NO* donor triggers formation of polarized acinar structure through a process termed ‘tumor reversion’.
The researchers showed that laminins, specifically LN1 and laminin-5 (LN5), help to produce NO*, which activates a number of specific molecules inside the malignant breast cells and restores the levels of the three miRNAs. Crucially, these microRNAs switch off two genes that are responsible for activating an enzyme that can degrade the LN5, thereby destroying their necessary signaling ability for the formation of the reciprocal regulatory loop. Turning off these enzymes protects the endogenously produced LN5 and the cells are able to form organized healthy structures, as well as allowing the reversion of the cancer cells to a normal morphology. “This suggests that if any of the positive or ‘good’ components of the loop were missing or overexpressed, the cells would again begin forming cancerous clumps,” says Bissell. “Reverting them, however, could help cancer cells to form ‘normal’ structures.”
In a related paper based partly on the Furuta/Bissell findings, published concurrently in eLife, Bissell and her collaborators contributed key information about the action of NO* in response to mechanical forces. Daniel Fletcher, Faculty Scientist in the BSE Division and Purnendu Chatterjee Professor and Chair of Bioengineering at UC Berkeley, led a team that studied the effect of transient external force on the healthy breast cells and malignant cells cultured in the 3D ECM gels from the Bissell Laboratory. When a brief transient force was applied to single malignant cells, they developed into more ‘normal’ structures.

Daniel Fletcher
Faculty Scientist, BSE Division, and Purnendu Chatterjee Professor and Chair of Bioengineering at UC Berkeley
Compression of these cells stimulated NO* production, and inhibiting NO* blocked this mechanically induced reversion. “It is quite remarkable that a transient physical force could produce reversion in these cells,” explains Bissell. “This has given us important insight into the quantity and quality of NO* that should be present in healthy cells. It is quite exciting also to find that the reason cancer cells are disorganized in 3D LN gels is because they do not make NO*. I am is now discussing with oncologists how to continue this research to find out whether these findings could have clinical relevance in cancer and other areas of biology including aging.”
Extracellular signals affect nuclear genes
These findings shed new light on how the extracellular matrix communicates with proteins in the nucleus to influence how single cells form breast tissues. It also shows that LNs are crucial for generating signals that regulate both form and function of specific tissues. The work from Bissell and Fletcher’s laboratories allows them to postulate that transient mechanical force could also play a role in signaling pathways.
“The understanding of how healthy and cancerous tissues form and re-form would help us understand the underlying mechanisms but also how to use this knowledge of dominance of context and microenvironment over form and function of the breast and other tissues for therapy,” says Bissell.
Other researchers who contributed to the first study under the leadership of Bissell and Furuta were Gang Ren, a research associate at the University of Toledo, and Jian-Hua Mao, senior scientist in the BSE Division. The second study was led by Fletcher, Bissell, and co-first authors Benjamin Ricca and Gautham Venugolupalan, both in Bioengineering at UC Berkeley. Other co-authors on the study were Kandice Tanner, a physicist and former fellow in Bissell lab (now an investigator in the Center for Cancer Research at the National Cancer Institute) who contributed preliminary information about the action of NO* in breast tissue as a function of pressure; and Walter Orellana, Clay Reber, and Douglas Brownfield, researchers in Bioengineering at UC Berkeley (Orellana and Brownfield are affiliated with the BSE Division, as well).
This work was supported at Berkeley Lab by the US Department of Defense, Breast Cancer Research Foundation (New York), and the National Cancer Institute.