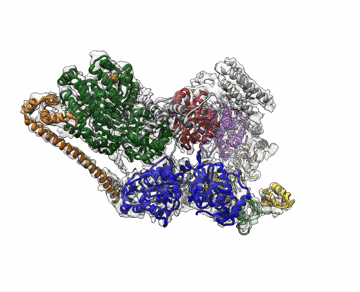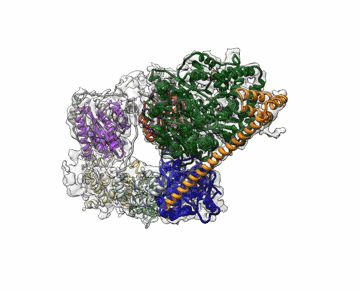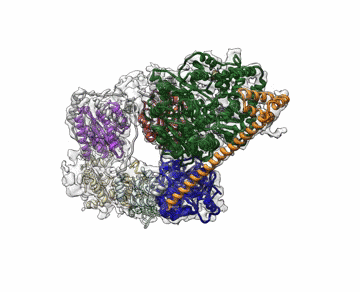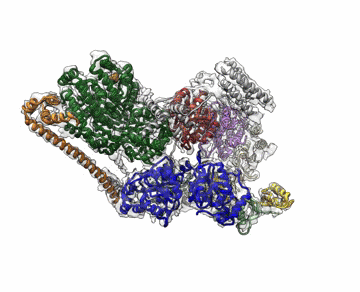
Molecular Biophysics and Integrated Bioimaging (MBIB) Division scientists led by Eva Nogales have resolved the 3-D structure of a critical human cellular protein complex involved in DNA transcription and repair at an unprecedented level of resolution. The complex, called transcription factor IIH (TFIIH), unzips the DNA double helix so that genes can be accessed and read. Malfunctions of the complex are associated with premature aging, cancer propensity, and a variety of other defects. One challenge with solving the structure of TFIIH has been that it exists in such minute amounts that it is difficult to produce and purify in large quantities. Moreover, once obtained, it may not form crystals suitable for X-ray diffraction. The researchers used cryo-electron microscopy (cryo-EM), a technique in which purified samples are flash-frozen at ultra cold temperatures, and which works even on very small quantities. “The fact that we resolved this protein structure from human cells makes this even more relevant to disease research,” said Nogales. Basil Greber, a postdoctoral fellow in Nogales’s lab, was first author on the study published in the journal Nature. Computational research scientist Pavel Afonine and MBIB Division Director Paul Adams also contributed to the project. Read more from the Berkeley Lab News Center.
Was this page useful?
Send