Whenever sugars are mentioned in relation to health and disease, it is in the context of metabolism and gaining calories. However, sugars have many other functions in our physiology and are found on cell surfaces and in extracellular matrix (ECM), forming an integral part of tissue microenvironment. Here they bind to their partner ligands, known as lectins, forming lectin-sugar interactions that have been known to play important roles in physiological and pathological contexts. In an article published and featured on the cover of Proceedings of the National Academy of Science (PNAS) last week, the laboratory of Distinguished Scientist Mina Bissell in the Biological Systems and Engineering Division of Lawrence Berkeley National Laboratory (Berkeley Lab), in collaboration with the research group of Professor Carolyn Bertozzi, now in the Stanford University Department of Chemistry, report exciting data and new insights into the roles a lectin, Galectin-1 (Gal-1), plays in mammary gland branching morphogenesis. This work also sheds some light on breast cancer progression.
Whenever sugars are mentioned in relation to health and disease, it is in the context of metabolism and gaining calories. However, sugars have many other functions in our physiology and are found on cell surfaces and in extracellular matrix (ECM), forming an integral part of tissue microenvironment. Here they bind to their partner ligands, known as lectins, forming lectin-sugar interactions that have been known to play important roles in physiological and pathological contexts. In an article published and featured on the cover of Proceedings of the National Academy of Science (PNAS) last week, the laboratory of Distinguished Scientist Mina Bissell in the Biological Systems and Engineering Division of Lawrence Berkeley National Laboratory (Berkeley Lab), in collaboration with the research group of Professor Carolyn Bertozzi, now in the Stanford University Department of Chemistry, report exciting data and new insights into the roles a lectin, Galectin-1 (Gal-1), plays in mammary gland branching morphogenesis. This work also sheds some light on breast cancer progression.
While Gal-1 is found both inside and outside the cell, scientists had gained more knowledge about what happens to Gal-1 outside the cell. When Gal-1 is secreted from the cell, it binds to lactose-containing sugars allowing the complex to regulate cell-cell and cell-ECM adhesion. This interaction between Gal-1 and its natural sugar ligand, N-acetyllactosamine (LacNAc), is inhibited when LacNAc is capped by another sugar known as α2,6-linked sialic acid (α2,6-SA). “We in the field of glycobiology have always been perplexed that Gal-1 and α2,6-SA are upregulated together in breast cancer,” says Bertozzi. “We would have expected to see that if there is more of one, the functions of the other would be less manifest.” In addition, researchers wondered about the spatial role of Gal-1 inside the cell, particularly when Ramray Bhat, then a postdoctoral fellow in Bissell’s group, found Gal-1 also inside the nucleus.
 “Ramray wanted to know what the molecule was doing in so many places,” recalled Bissell (pictured, right). With her help, Bhat formed a team that included researchers from University of California (UC), Berkeley; UC Davis and Stanford University. Together they uncovered a novel function for Gal-1 in mammary gland development in mice. “We knew that normal cells of branching mammary glands and malignant breast cancer cells share the ability to migrate and invade through their surroundings,” Bissell said. “But we didn’t know how extracellular sugars and their binding partners are involved in this process.”
“Ramray wanted to know what the molecule was doing in so many places,” recalled Bissell (pictured, right). With her help, Bhat formed a team that included researchers from University of California (UC), Berkeley; UC Davis and Stanford University. Together they uncovered a novel function for Gal-1 in mammary gland development in mice. “We knew that normal cells of branching mammary glands and malignant breast cancer cells share the ability to migrate and invade through their surroundings,” Bissell said. “But we didn’t know how extracellular sugars and their binding partners are involved in this process.”
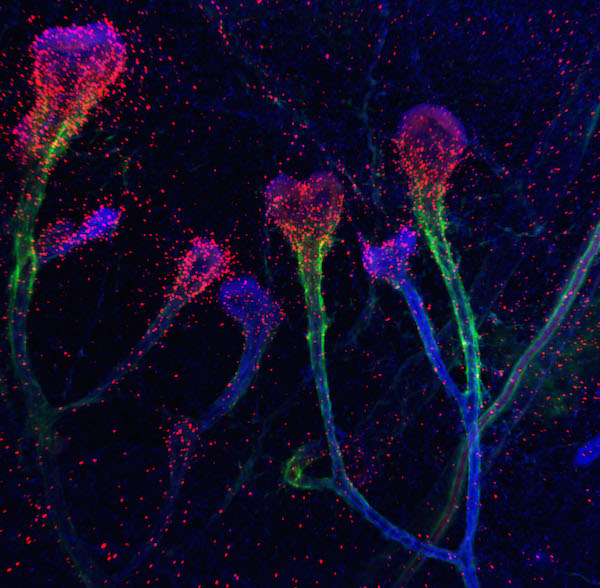
Combining years of expertise in cell and cancer biology and the Bissell laboratory’s established three-dimensional organotypic cultures with the cutting edge glycochemistry tools developed by Bertozzi’s group, the researchers showed that Gal-1, traditionally thought to play extracellular roles, translocates to the nucleus where it spurs migration of mammary cells. Bhat (pictured, below), now an assistant professor at the Indian Institute of Science in Bangalore and co-first author of the PNAS article, said, “What  was even more surprising was that this translocation is regulated by sugars on the cell surface.” The scientists saw a correlation between the levels of α2,6-SA and Gal-1: high levels of α2,6-SA meant stronger Gal-1 translocation to the nucleus. This was seen in the cells at the forefront of the invasion of the mammary cells into the fat pad. The cells within the already formed ducts behind the invasive front showed higher levels of LacNAc that were uncapped with α2,6-SA resulting in lower nuclear Gal-1 levels and hence no migration. “When translocated to the nucleus,” Bhat continued, “Gal-1 is necessary for the branching migration of epithelia during mammary gland development. Since we knew that both Gal-1 and α2,6-SA are upregulated in breast cancer, we wanted to see how this interaction operated in the malignant context.”
was even more surprising was that this translocation is regulated by sugars on the cell surface.” The scientists saw a correlation between the levels of α2,6-SA and Gal-1: high levels of α2,6-SA meant stronger Gal-1 translocation to the nucleus. This was seen in the cells at the forefront of the invasion of the mammary cells into the fat pad. The cells within the already formed ducts behind the invasive front showed higher levels of LacNAc that were uncapped with α2,6-SA resulting in lower nuclear Gal-1 levels and hence no migration. “When translocated to the nucleus,” Bhat continued, “Gal-1 is necessary for the branching migration of epithelia during mammary gland development. Since we knew that both Gal-1 and α2,6-SA are upregulated in breast cancer, we wanted to see how this interaction operated in the malignant context.”
Using an in-house culture model of cancer progression in the Bissell lab, the scientists showed that spatiotemporal dynamics of Gal-1, LacNAc and α2,6-SA witnessed in migrating and branching mammary gland cells are seen also in invading malignant breast epithelia. Co-first author Brian Belardi, now a postdoctoral fellow at UC Berkeley, said, “Our findings raise new questions for future research, such as how Gal-1 gets to the nucleus and how once there it influences signaling processes necessary for branching and invasion.”
The researchers believe the results of this study suggest a novel direction in the design of Gal-1-based molecular therapeutics in breast cancer. Instead of targeting the interaction between Gal-1 and its extracellular ligands, therapeutic agents could be designed to bind to Gal-1 and act as extracellular sinks, preventing its nuclear translocation.
Mina Bissell has led her group to show repeatedly that normal mechanisms of organ development when redeployed in a deregulated manner, can lead to a malignancy within the same organ. “This study contributes to our understanding of how structural sugars and their binding partners are specially orchestrated to contribute to organogenesis. In the process it has solved an intriguing paradox in breast cancer research with therapeutic implications,” says Bissell.