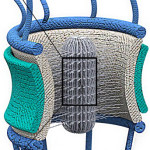
Scientists have gained insight on how some molecules are allowed to enter and exit the nucleus while keeping other molecules out. Their research, led by Mohammad Mofrad in the Molecular Biophysics & Integrated Bioimaging Division, is a step forward in building a more comprehensive understanding of nuclear pore complex function, which has numerous implications in health and disease.  Now, as reported November 6 in the journal Scientific Reports, Mofrad’s team found that the proteins’ cargo-transporting ability is likely due to specific features encoded in their sequences. Protein sequences are primarily composed of amino acids, and in FG Nups proteins, the scientists found different types of amino acids arranged just so within their sequences. This arrangement of amino acids appears to form a network inside the nuclear pore complex that is optimized for one purpose only: the extremely efficient transport of molecular cargo into and out of the nucleus. In addition to the potential impacts for improving health and treating disease, Mofrad stated that these results can set a basis for the design of nuclear pore complex-inspired nanopores for filtering and separation technologies. Read more at Berkeley Lab News Center.
Now, as reported November 6 in the journal Scientific Reports, Mofrad’s team found that the proteins’ cargo-transporting ability is likely due to specific features encoded in their sequences. Protein sequences are primarily composed of amino acids, and in FG Nups proteins, the scientists found different types of amino acids arranged just so within their sequences. This arrangement of amino acids appears to form a network inside the nuclear pore complex that is optimized for one purpose only: the extremely efficient transport of molecular cargo into and out of the nucleus. In addition to the potential impacts for improving health and treating disease, Mofrad stated that these results can set a basis for the design of nuclear pore complex-inspired nanopores for filtering and separation technologies. Read more at Berkeley Lab News Center.