 The Ebola virus outbreak in West Africa has claimed over 110 lives and more than 170 suspected or confirmed cases have been reported. While there is no known cure, basic research is providing insight into the action of the virus. Zachary Bornholdt and Erica Ollman Saphire from The Scripps Research Institute spearheaded a team of researchers from the University of Wisconsin-Madison, University of Tokyo, and the Japan Science and Technology Agency, studying an Ebola virus protein. The Ebola virus genome encodes for 8 proteins, one of which is VP40, a multifunctional protein with critical roles at different stages of the virus life cycle. To elucidate the VP40 structure and its related function, they crystallized the protein and collected data at three light sources, including the Berkeley Center for Structural Biology (Beamline 5.0.2) at the Advanced Light Source. The researchers analyzed the X-ray data using PHENIX, automated crystallography software for determining macromolecular structure, developed under the direction of Paul Adams in the Physical Biosciences Division.
The Ebola virus outbreak in West Africa has claimed over 110 lives and more than 170 suspected or confirmed cases have been reported. While there is no known cure, basic research is providing insight into the action of the virus. Zachary Bornholdt and Erica Ollman Saphire from The Scripps Research Institute spearheaded a team of researchers from the University of Wisconsin-Madison, University of Tokyo, and the Japan Science and Technology Agency, studying an Ebola virus protein. The Ebola virus genome encodes for 8 proteins, one of which is VP40, a multifunctional protein with critical roles at different stages of the virus life cycle. To elucidate the VP40 structure and its related function, they crystallized the protein and collected data at three light sources, including the Berkeley Center for Structural Biology (Beamline 5.0.2) at the Advanced Light Source. The researchers analyzed the X-ray data using PHENIX, automated crystallography software for determining macromolecular structure, developed under the direction of Paul Adams in the Physical Biosciences Division.
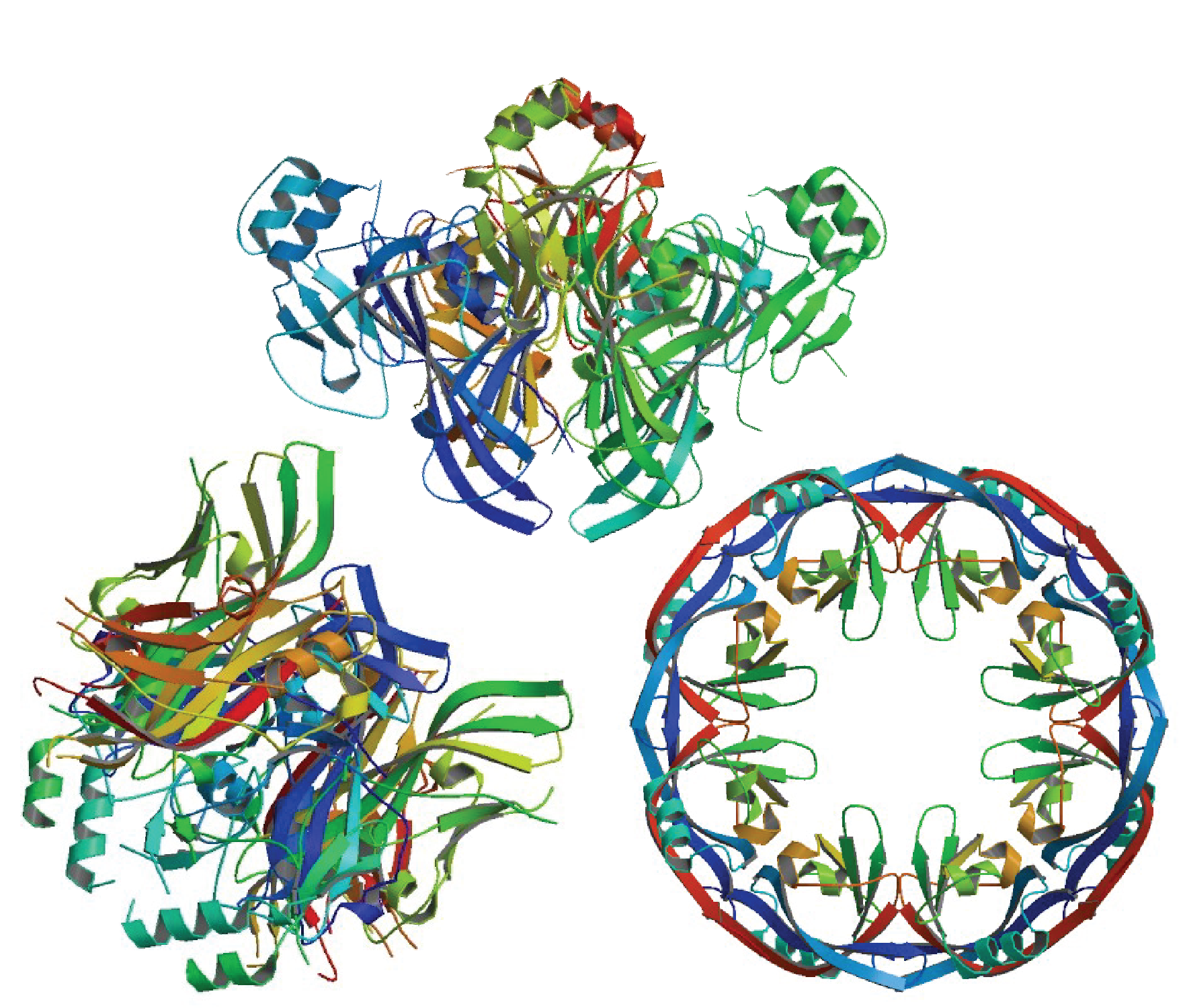
In a study published in Cell, Bornholdt and colleagues report that VP40 has three different conformations: a dimer, hexamer, and octamer (pictured; Bornholdt, et al., 2013). Further studies of these multiple forms indicate that this viral protein assumes different conformations depending on the function being performed. When a dimer, VP40 interacts with other VP40 dimers to form a linear filament that is essential for building the shell of the virus. Through electrostatic interaction and conformational changes, the dimer rearranges into a hexamer responsible for building and budding Ebola virus virions. As an eight-member ring, assembled from four dimers, VP40 binds RNA, the genetic material of the Ebola virus, and acts to control viral transcription in infected cells.
Much like the multi-functional protein itself, these results accomplish more than one goal. First, this work provides evidence to support the theory that the proteins of other viruses may be performing multiple functions. Second, these findings reinforce the desirability of VP40 as a target for anti-Ebola virus drugs. The structure-shifting nature of this protein allows for either targeting by more than one drug or upsetting the balance of the different protein conformations, thereby impeding the viral life cycle. Regardless of the approach taken, this work highlights areas that can be exploited to decrease the virulence of this deadly Ebola virus.