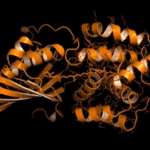Engineering artificial nanofactories modeled on bacterial microcompartments (BMCs) will require a chain of “logistical” vehicles to deliver the products. Making progress on that front, scientists affiliated with Berkeley Lab’s Molecular Biophysics and Integrated Bioimaging Division and the MSU-DOE Plant Research Laboratory have detailed the structure and function of a BMC-associated protein involved in electron transfer, a fundamental part of the assembly line that leads to the production of chemical compounds. The study, published in the journal Biochemistry, was led by Cheryl Kerfeld; Marcus Sutter also collaborated on the project. Read more from the MSU-DOE Plant Research Lab.
New Addition to Family of Light-responsive Proteins
A study led by Cheryl Kerfeld, with colleagues from Biosciences’ Molecular Biophysics and Integrated Bioimaging (MBIB) Division, as well as her MSU-DOE Plant Research group at Michigan State University, made the cover of the August 2017 issue of Nature Plants. Matthew Melnicki, Markus Sutter, and Fei Cai of MBIB contributed to the study, which characterized a recently identified member of the orange carotenoid family of proteins (OCPs). These proteins change conformation in response to ambient light conditions to protect the host cyanobacteria from harmful exposure. Compared to the canonical exemplar, OCP1, the new OCP, called OCP2, requires relatively higher light intensity for activation, but it reacts faster than OCP1. The goal of Kerfeld’s OCP research is to understand how the various members of the family work, and use that knowledge to engineer the protein for applications in renewable energy and medicine. Read more from the MSU-DOE Plant Research Lab.
Novel Orange Carotenoid Proteins Shed Light on Evolution of Cyanobacteria Photoprotection
Research led by Cheryl Kerfeld, with members of her group in Berkeley Lab Biosciences’ Molecular Biophysics and Integrated Bioimaging (MBIB) Division, as well as her MSU-DOE Plant Research group at Michigan State University, has identified and characterized a new, functionally distinct member of the Orange Carotenoid Protein (OCP) family. The OCP complex enables chromatically acclimating blue-green algae to avoid cellular damage and growth inhibition in conditions of high light or nutrient stress.
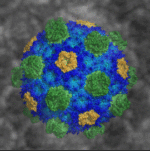
An Atomic-Level Snapshot of Bacterial Microcompartment Architecture
Researchers led by Berkeley Lab Biosciences’ Cheryl Kerfeld (MBIB), who holds a joint appointment at the MSU-DOE Plant Research Laboratory, have provided the first atomic-level resolution picture of an intact bacterial microcompartment (BMC) shell. The intact shell and component proteins from the saltwater bacterium Haliangium ochraceum were crystallized at Berkeley Lab; X-ray diffraction data were collected at the Lab’s Advanced Light Source (ALS) and at the Stanford Synchrotron Radiation Lightsource. Details of the BMC shell’s structure and composition were published in the journal Science.
How to Build an Artificial Nano-factory to Power Our Futures
Many bacteria contain structures called carboxomes that act as mini factories for various purposes, such as building sugar from carbon dioxide through photosynthesis. Researchers affiliated with Berkeley Lab’s Molecular Biophysics and Integrated Bioimaging (MBIB) Division and the Department of Energy Plant Research Laboratory and Department of Biochemistry and Molecular Biology at Michigan State University are studying how these factories are constructed and how they function. In a key step toward identifying the essential building blocks, Manuel Sommer and Cheryl Kerfeld have analyzed more than 200 sets of genes from different cyanobacteria—also known as blue-green algae—that contain carboxysomes. The work lays the foundation for designing new kinds of factories that could produce synthetic materials, such as fragrances or the precursors for green fuels. The study was published in the Journal of Experimental Botany. Read more from the MSU-DOE Plant Research Lab.
Was this page useful?