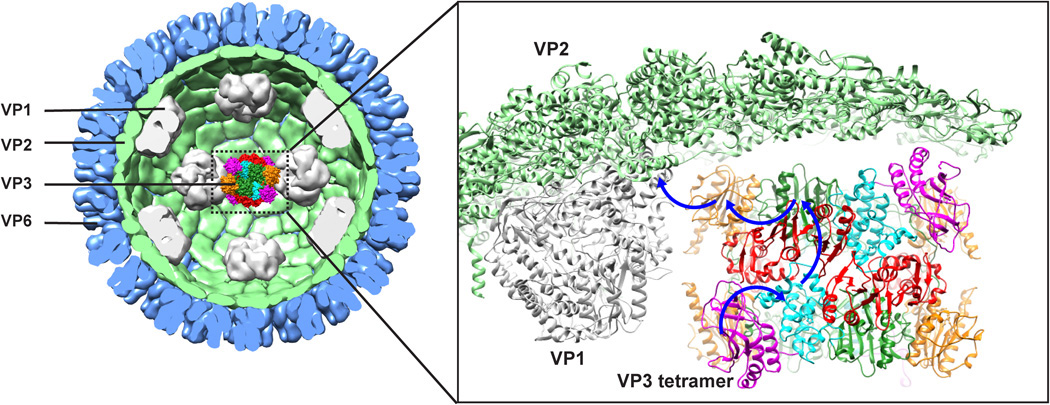
Rotavirus, a major cause of infantile gastroenteritis, is responsible for the deaths of about 200,000 children per year, mostly in developing countries. Of the six proteins that form the rotavirus particle, only one—viral protein 3 (VP3)—had a structure that remained unsolved after 30 years. Scientists had deduced that VP3 was necessary for “capping” the viral messenger RNA (mRNA), enabling it to mimic the host’s capped RNA and thus evade immune response.
Combining cryo-electron microscopy, biochemical assays, and protein crystallography at Advanced Light Source (ALS) Beamline 5.0.2 (part of the Berkeley Center for Structural Biology), researchers from the Baylor College of Medicine discovered that VP3 incorporates in one place all the enzymatic activities required to effectively cap rotavirus mRNA, making it unique among viral-capping enzymes.
Proposed model for mRNA capping. The blue arrows in the inset indicate the possible path for the nascent transcript during the capping process.
This VP3 structure, with such a confluence of enzymatic activities, provides a rational platform for designing antivirals to counter rotavirus infection.
Read the ALS Science Brief.