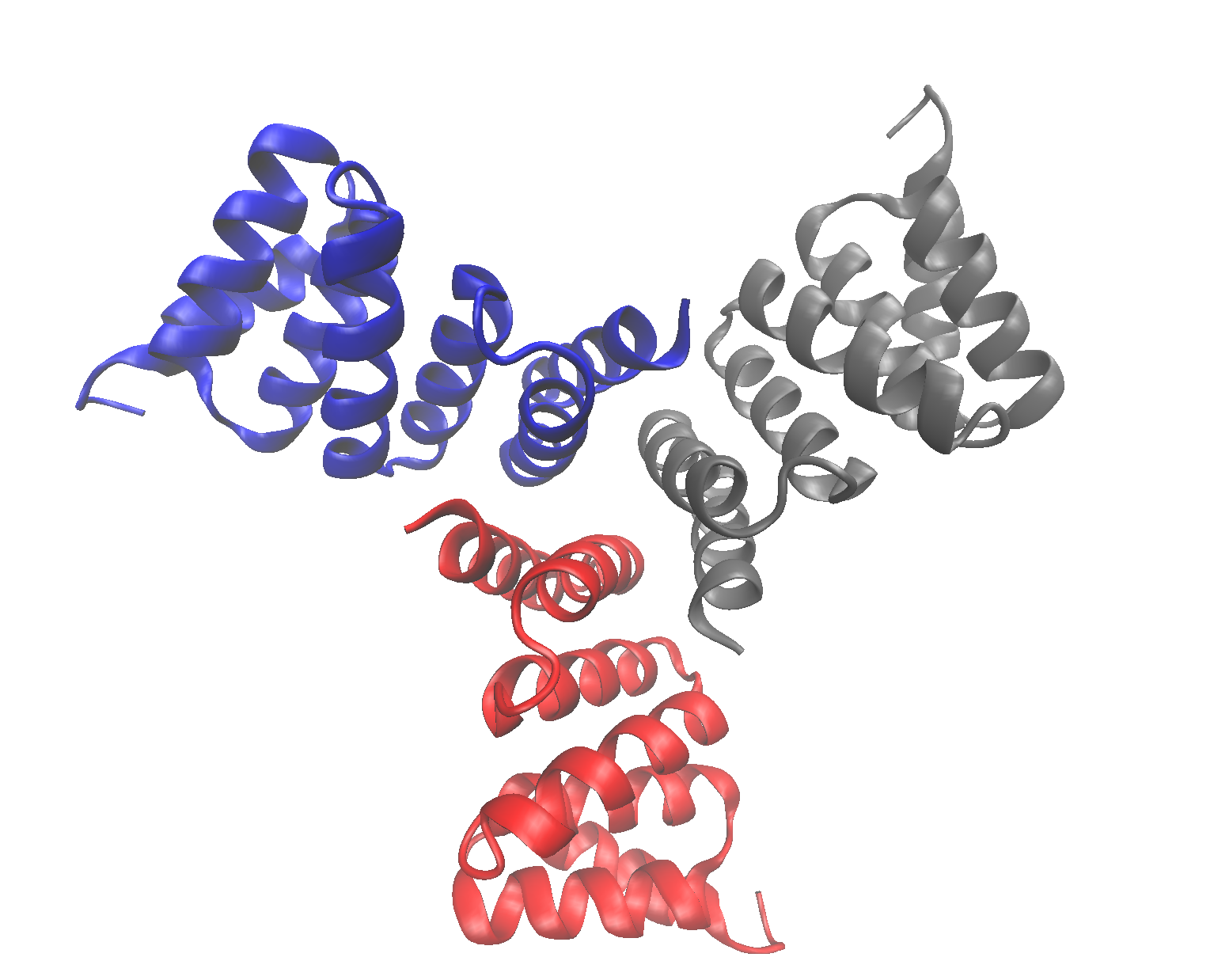
Cyclic proteins that assemble from multiple identical subunits (homo-oligomers) play key roles in many biological processes, including enzymatic catalysis and function and cell signaling. Researchers in the Molecular Biophysics and Integrated Bioimaging (MBIB) Division worked with University of Washington’s David Baker, who led a team to design in silico and crystallize self-assembling cyclic homo-oligomer proteins.
A strategy was developed, described in an April 2017 Nature Chemistry publication, which designs interfaces onto idealized proteins aimed to direct their assembly into multimeric complexes. Berkeley Lab researchers used structural characterization – both X-ray crystallography and small angle X-ray scattering (SAXS), to show that many of the designs adopted the target oligomerization state and predicted structure. Not only does their work demonstrate that scientists have a basic understanding of what determines oligomerization, but that they could design proteins with tunable shape, size and symmetry for a variety of biological applications.
Some of the X-ray crystallography work included in the paper was performed under the auspices of the Crystallography Collective program at the Advanced Light Source, which is run by Research Scientist Banumathi Sankaran. Fellow research scientist Henrique Pereira crystallized the proteins designed by the University of Washington researchers. Sankaran and Peter Zwart, MBIB staff scientist, collected crystallographic data on beamline 5.0.2 in the Berkeley Center for Structural Biology and solved the structures. To gain information about the designed protein dynamics, Kathryn Burnett and Greg Hura of MBIB performed SAXS on the SIBYLS beamline.
Pereira, Sankaran, and Zwart have been co-authors on several papers with David Baker and his team, all of which follow a theme of protein design and structure validation. “Cyclic homooligomers play important roles in biological function,” said Sankaran. “Here we have another synthetic design that was proven to match the computational design with both small angle solution studies and X-ray scattering.” This work, along with the curved beta sheet and trimeric metalloprotein designs previously described, widen the possibilities for developing novel therapeutics and biomaterials.